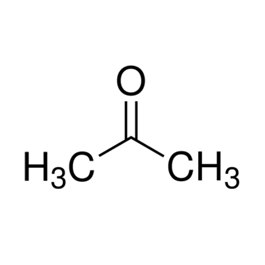
Dmitrievsky chemical plant offers to buy technical acetone CAS No 67-64-1. We have all the required licenses for the usage of chemically hazardous production facilities. Currently it's offered at bargain price technical acetone and comfortable conditions of payment and delivery of goods. You can simply buy wholesale acetone, by calling us.
Famous home solvent is used in industrial applications to dissolve natural resins, oils, cellulose diacetate, polystyrene, epoxy resins, vinyl chloride copolymers, polyacrylates, chlorinated rubber, the component is considered almost all mixed solvents.
Technical acetone included in the compound of solvents: 646, 647, 648, P-4, P-4A-R 5-R 5A etc. In pure acetone can be used to dilute the enamel EP-437, EP-438, CS-436, CS-510, primers EP-0263 and EP-0199.
Butyl acetate CAS-No 123-86-4 dissolve cellulose esters, oils, fats, chlorinated rubber, vinyl polymers, resins carbinol. Adding butyl acetate along with a small amount of butyl alcohol prevents whitening lacquer films.
Due to the wonderful ability to dissolve varnish, butyl acetate is widely used in cosmetic female nail polish remover. Its use is considerably safer than the use of acetone. But as the cost of butyl acetate almost twice as expensive, many manufacturers add blended acetone, causing considerable damage to the skin. In the EU countries such adding is strictly prohibited, and we use them no regulated. Therefore, choosing a wash varnish, carefully sniffing the purchased goods. Caramel flavor is the card safer butyl acetate. Sharp clubbing nose smell, not even masked fragrances, gives an extremely dangerous for the skin and nails acetone.
There are four isomers of butanol:
- Primary or normal butyl alcohol CAS-No 71-36-3(n-butanol) (1-butanol),
- normal isobutanol CAS-No 78-83-1 (normal secondary butyl alcohol) (2-methyl-1-propanol)
- sec-butyl alcohol (isobutyl alcohol) (2-butanol)
- tert-butyl alcohol (t-butanol), (trimethyl) (2-methyl-2-propanol).
The toxicity of butanol is relatively low, but highest among younger alcohol. Ingestion butanol produces an effect similar to the effect of the use of ethanol. Butanol is always found in small amounts in various alcoholic beverages. Often butanol obtained from fluids used illegal entrepreneurs as a surrogate alcoholic beverage. Butanol concentration in air of 0.01 % or does not affect the human body, while 0.02 % causes an inflammation of the cornea.