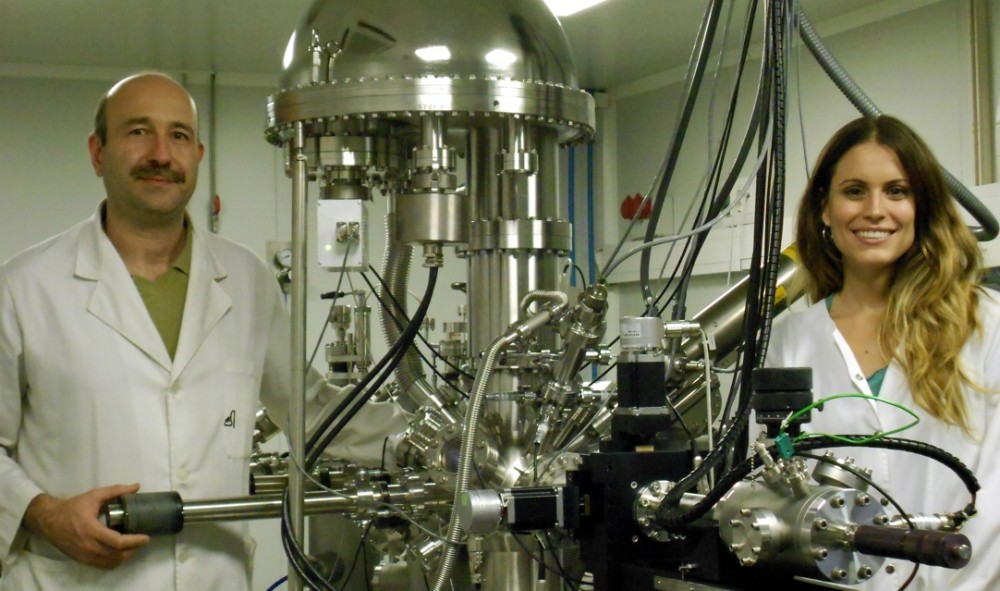
A team led by Jordi Llorca, a professor at the Universitat Politècnica de Catalunya (UPC), has discovered that atoms react differently depending on the characteristics of the catalyst that is used. The study, which is a very important step forward in the design of new catalysts with applications in the field of energy, involved the UPC doctoral student Núria Jiménez Divins, researchers Carlos Escudero and Virginia Pérez-Dieste from the ALBA synchrotron, where part of the experiment was carried out, and researcher Inma Angurell from the University of Barcelona (UB), who synthesised the nanoparticles that were used in the experiment.
Catalysts, which are used in 95% of industrial processes, can eliminate pollution from gases emitted by vehicles with combustion engines. They are substances that speed up chemical reactions and the human body has hundreds of them in the form of enzymes. From the point of view of energy, the role of a catalyst is to reduce the amount of energy required to trigger these reactions.
A team of researchers led by Jordi Llorca from the Nanoengineering Research Centre (CRnE) and the Institute of Energy Technologies (INTE) - both of which belong to the UPC - has studied how atoms move in a real catalyst and has demonstrated that they react differently depending on the type of support being used. This research opens the door to designing new custom-made catalysts for energy and industrial applications and for the removal of pollutant gases.
The catalyst chosen by the researchers contained metal (rhodium and palladium) nanoparticles prepared by the Dendrimers and Molecular Polygons Group at the UB. The nanoparticles were fixed to a ceria support. This catalyst is very effective at producing hydrogen, a product that could replace fossil fuels before they are depleted and allow the current energy model to be changed in favour of a more sustainable and environmentally friendly one.
Jordi Llorca explains that the results of the study pave the way for obtaining hydrogen in the most efficient way possible, that is, from water and ethanol, the latter of which is a renewable, inexpensive resource that is easily obtained from forestry and agricultural waste. As Lorca says, a metaphor for understanding this more efficient process would be finding the best way to cross a mountain: "The shortest route is to climb up one side and then down the other, but this option is the one that requires the greatest amount of energy. If we find a better way to cross the mountain, although it may seem longer it will require less energy and therefore we will get across the mountain faster."
One step in finding the way is to learn how atoms and nanoparticles really behave in a catalyst and whether they always behave in the same way. For this experiment, the researchers used the CIRCE beamline of the ALBA synchrotron located in Cerdanyola del Vallès, and specifically near ambient pressure photoemission (NAPP). NAPP was developed by a research group led by Professor Miquel Salmerón in early 2000 at the Lawrence Berkeley National Laboratory in California, USA. One of a kind in Spain and available at only eight synchrotrons worldwide, the experimental NAPP station at the ALBA synchrotron became operational in September 2013 and this was its first experiment.
Up until then, researchers had been able to ascertain what happens when the ethanol and water molecules are heated to 550 degrees Celsius, in the X-ray photoelectron spectroscopy chamber at the UPC's CRnE. Thanks to the ALBA synchrotron, however, researchers were able to more accurately pinpoint the movement of atoms in the nanoparticles during chemical reactions (i.e. in operando) and found that these nanoparticles behave differently depending on the characteristics of the catalytic support, which can affect their composition, form and nanostructure.
As Jordi Llorca says, "the nanoparticles know where they are supported and react accordingly". This discovery, he explains, "paves the way for custom-made catalysts that are more efficient because they can be developed or adapted according to the process for which they are required".
In the case of hydrogen, the research team discovered that to produce it the atoms in the catalyst need to be in certain positions. These positions allow electrons to be exchanged between the metal nanoparticles and the ceria support appropriately when they break and form new chemical bonds to produce hydrogen.
In vehicles that use combustion engines (cars, motorcycles, planes, ships, etc.) with ceria-supported catalysts, new nanostructures could be designed or existing ones adapted to make them more energy efficient.
Source: Universitat Politècnica de Catalunya (UPC)